Journal Description
Current Oncology
Current Oncology
is an international, peer-reviewed, open access journal published online by MDPI (from Volume 28 Issue 1-2021). Established in 1994, the journal represents a multidisciplinary medium for clinical oncologists to report and review progress in the management of this disease. The Canadian Association of Medical Oncologists (CAMO), the Canadian Association of Psychosocial Oncology (CAPO), the Canadian Association of General Practitioners in Oncology (CAGPO), the Cell Therapy Transplant Canada (CTTC), the Canadian Leukemia Study Group (CLSG) and others are affiliated with the journal and their members receive a discount on the article processing charges.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, SCIE (Web of Science), PubMed, MEDLINE, PMC, Embase, and other databases.
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 18 days after submission; acceptance to publication is undertaken in 2.8 days (median values for papers published in this journal in the second half of 2023).
- Recognition of Reviewers: APC discount vouchers, optional signed peer review, and reviewer names published annually in the journal.
Impact Factor:
2.6 (2022);
5-Year Impact Factor:
2.9 (2022)
Latest Articles
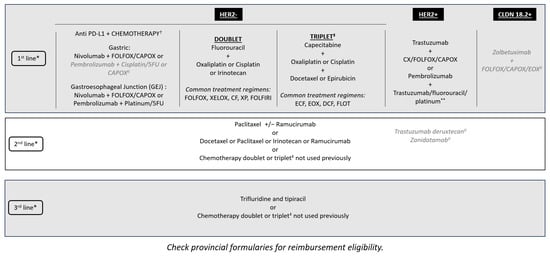
Best Practices for Managing Patients with Unresectable Metastatic Gastric and Gastroesophageal Junction Cancer in Canada
Curr. Oncol. 2024, 31(5), 2552-2565; https://doi.org/10.3390/curroncol31050191 (registering DOI) - 30 Apr 2024
Abstract
►
Show Figures
Gastric cancer (GC) is one of the most common types of cancer and is associated with relatively low survival rates. Despite its considerable burden, there is limited guidance for Canadian clinicians on the management of unresectable metastatic GC and gastroesophageal junction cancer (GEJC).
[...] Read more.
Gastric cancer (GC) is one of the most common types of cancer and is associated with relatively low survival rates. Despite its considerable burden, there is limited guidance for Canadian clinicians on the management of unresectable metastatic GC and gastroesophageal junction cancer (GEJC). Therefore, we aimed to discuss best practices and provide expert recommendations for patient management within the current Canadian unresectable GC and GEJC landscape. A multidisciplinary group of Canadian healthcare practitioners was assembled to develop expert recommendations via a working group. The often-rapid progression of unresectable GC and GEJC and the associated malnutrition have a significant impact on the patient’s quality of life and ability to tolerate treatment. Hence, recommendations include early diagnosis, identification of relevant biomarkers to improve personalized treatment, and relevant support to manage comorbidities. A multidisciplinary approach including early access to registered dietitians, personal support networks, and palliative care services, is needed to optimize possible outcomes for patients. Where possible, patients with unresectable GC and GEJC would benefit from access to clinical trials and innovative treatments.
Full article
Open AccessReview
Optimizing Access to Unrelated Donors in Canada: Re-Examining the Importance of Donor Factors on Outcomes Following Hematopoietic Cell Transplantation
by
Gaganvir Parmar, Matthew D. Seftel, Kathy Ganz, John Blake, Jelena L. Holovati and David S. Allan
Curr. Oncol. 2024, 31(5), 2542-2551; https://doi.org/10.3390/curroncol31050190 (registering DOI) - 30 Apr 2024
Abstract
HLA-matched allogeneic hematopoietic cell transplantation (HCT) is a curative therapy for many patients. Unrelated HLA-matched donors are the most frequently used donor for HCT. When more than one donor transplant option is available, transplant centers can select donors based on non-HLA factors. With
[...] Read more.
HLA-matched allogeneic hematopoietic cell transplantation (HCT) is a curative therapy for many patients. Unrelated HLA-matched donors are the most frequently used donor for HCT. When more than one donor transplant option is available, transplant centers can select donors based on non-HLA factors. With improved ability to prevent and treat immune complications, such as graft-versus-host disease and infections, it may be possible to proceed more often using HLA-mismatched donors, allowing greater consideration of non-HLA factors, such as donor age, CMV serostatus, and ABO blood group matching, which have demonstrated important impacts on transplant outcomes. Additional factors to consider are donor availability rates and the usage of domestic donors to optimize outcomes. A review of non-HLA factors and considerations on the selection of optimal unrelated donors for HCT are provided within this updated current context.
Full article
(This article belongs to the Section Cell Therapy)
Open AccessArticle
Beauty Therapy to Support Psychosocial Recovery from Oncological Care: A Qualitative Research on the Lived Experience of Women with Breast Cancer Treated with Chemotherapy
by
Denise Vagnini, Massimo Maria Grassi, Francesco Valenti, Emilio Bombardieri and Emanuela Saita
Curr. Oncol. 2024, 31(5), 2527-2541; https://doi.org/10.3390/curroncol31050189 (registering DOI) - 30 Apr 2024
Abstract
During the oncological care path, breast cancer patients treated with chemotherapy suffer from a number of psycho-physical changes, and appearance-related side effects are among the primary determinants of psychosocial impairment. Appropriate interventions are needed due to the fact that treatment-induced transformations have been
[...] Read more.
During the oncological care path, breast cancer patients treated with chemotherapy suffer from a number of psycho-physical changes, and appearance-related side effects are among the primary determinants of psychosocial impairment. Appropriate interventions are needed due to the fact that treatment-induced transformations have been associated with a decline in overall quality of life, interpersonal and sexual difficulties, and adverse effects on therapeutic adherence. In the framework of integrative oncology, beauty therapy is an affordable and straightforward intervention that could be used in the clinical management of breast cancer side effects. This study aims to comprehend the emotional and lived experiences of women undergoing chemotherapy after a brief beauty therapy intervention with licensed beauticians. The Interpretative Phenomenological Analysis was used as a methodological guideline. Sixteen women were purposefully recruited in a day hospital of a cancer unit, where the beauty therapy was implemented. At the end of the intervention, data were gathered using a semi-structured interview with open-ended questions. A thematic analysis was performed on verbatim transcriptions. Findings support the proposal of beauty therapy for patients undergoing chemotherapy. Assuming a relational viewpoint, beauty therapy could improve patients’ feelings about themselves and the way they feel about others, even if they do not declare a specific interest in their outward appearance.
Full article
(This article belongs to the Special Issue Body Image among Cancer Survivors: New Theoretical Frameworks and Psychological Interventions)
►▼
Show Figures

Figure 1
Open AccessReview
Impact of PET/CT Imaging with FDG in Locally Advanced Cervical Carcinoma—A Literature Review
by
Ottó Molnar, Oreste Mihai Straciuc, Simona Mihuțiu and Liviu Lazăr
Curr. Oncol. 2024, 31(5), 2508-2526; https://doi.org/10.3390/curroncol31050188 (registering DOI) - 29 Apr 2024
Abstract
Positron emission tomography (PET) and computed tomography (CT) have evolved as a pivotal diagnostic modality in the field of oncology. With its increasing application in staging and ready availability, it becomes imperative for committed radiation oncologists to possess a complete analysis and understanding
[...] Read more.
Positron emission tomography (PET) and computed tomography (CT) have evolved as a pivotal diagnostic modality in the field of oncology. With its increasing application in staging and ready availability, it becomes imperative for committed radiation oncologists to possess a complete analysis and understanding of integration of molecular imaging, which can be helpful for radiation planning, while also acknowledging its possible limitations and challenges. A significant obstacle lies in the synthesis and design of tumor-specific bmolecules for diagnosing and treating cancer. The utilization of radiation in medical biochemistry and biotechnology, encompassing diagnosis, therapy, and control of biological systems, is encapsulated under the umbrella term “nuclear medicine.” Notably, the application of various radioisotopes in pharmaceutics has garnered significant attention, particularly in the realm of delivery systems for drugs, DNA, and imaging agents. The present article provides a comprehensive review of use of novel techniques PET and CT with major positron-emitting radiopharmaceuticals currently in progress or utilized in clinical practice with their integration into imaging and radiation therapy.
Full article
(This article belongs to the Section Gynecologic Oncology)
Open AccessReview
Sublobar Resection of Non-Small-Cell Lung Cancer: Wedge Resection vs. Segmentectomy
by
Kyeong Ri Yu and Walker A. Julliard
Curr. Oncol. 2024, 31(5), 2497-2507; https://doi.org/10.3390/curroncol31050187 (registering DOI) - 29 Apr 2024
Abstract
Lung cancer is the most common cause of cancer death. The mainstay treatment for non-small-cell lung cancer (NSCLC), particularly in the early stages, is surgical resection. Traditionally, lobectomy has been considered the gold-standard technique. Sublobar resection includes segmentectomy and wedge resection. Compared to
[...] Read more.
Lung cancer is the most common cause of cancer death. The mainstay treatment for non-small-cell lung cancer (NSCLC), particularly in the early stages, is surgical resection. Traditionally, lobectomy has been considered the gold-standard technique. Sublobar resection includes segmentectomy and wedge resection. Compared to lobectomy, these procedures have been viewed as a compromise procedure, reserved for those with poor cardiopulmonary function or who are poor surgical candidates for other reasons. However, with the advances in imaging and surgical techniques, the subject of sublobar resection as a curative treatment is being revisited. Many studies have now shown segmentectomy to be equivalent to lobectomy in patients with small (<2.0 cm), peripheral NSCLC. However, there is a mix of evidence when it comes to wedge resection and its suitability as a curative procedure. At this time, until more data can be found, segmentectomy should be considered before wedge resection for patients with early-stage NSCLC.
Full article
(This article belongs to the Special Issue New Advances in the Treatment of Resectable Non-small Cell Lung Cancer)
Open AccessArticle
Evaluation of Unsolicited Feedback from Patients with Cancer and Their Families as a Strategy to Improve Cancer Care Delivery
by
Parvaneh Fallah, Lucas Clemons, Michelle Bradbury, Lisa Vandermeer, Mark Clemons, Julie Renaud and Marie-France Savard
Curr. Oncol. 2024, 31(5), 2488-2496; https://doi.org/10.3390/curroncol31050186 (registering DOI) - 28 Apr 2024
Abstract
Background: Unsolicited patient feedback (compliments and complaints) should allow the healthcare system to address and improve individual and overall patient, family, and staff experiences. We evaluated feedback at a tertiary cancer centre to identify potential areas for optimizing care delivery. Methods: unsolicited feedback
[...] Read more.
Background: Unsolicited patient feedback (compliments and complaints) should allow the healthcare system to address and improve individual and overall patient, family, and staff experiences. We evaluated feedback at a tertiary cancer centre to identify potential areas for optimizing care delivery. Methods: unsolicited feedback submitted to the Patient Relations Department, relating to the Divisions of Medical and Radiation Oncology, at the Ottawa Hospital, was analyzed. Results: Of 580 individual reports submitted from 2016 to 2022, patient demographics were available for 97% (563/580). Median patient age was 65 years (range 17–101), and 53% (301/563) were female. The most common cancer types were breast (127/545, 23%) and gastrointestinal (119/545, 22%) malignancies, and most (64%, 311/486) patients had metastatic disease. Feedback was submitted mainly by patients (291/579, 50%), and predominantly negative (489/569, 86%). The main reasons for complaints included: communication (29%, 162/566) and attitude/conduct of care (28%, 159/566). While feedback rates were initially stable, an increase occurred from 2019 to 2021. Conclusions: Unsolicited feedback remains mostly negative, and relates to physician communication. If we are to drive meaningful changes in care delivery, more standardized means of assessing feedback and implementation strategies are needed. In addition, in an era of increased healthcare provider burnout, strategies to enhance formal positive feedback are also warranted.
Full article
(This article belongs to the Special Issue The 30th Anniversary of Current Oncology: Perspectives in Clinical Oncology Practice)
►▼
Show Figures
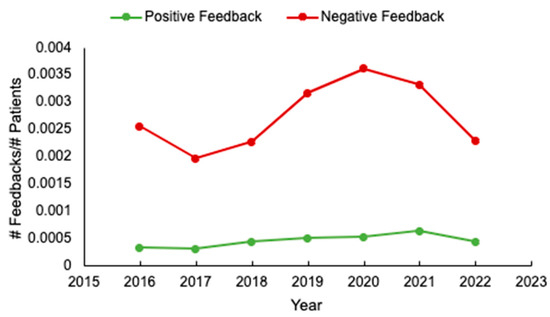
Figure 1
Open AccessCommunication
The Immune Response of Cutaneous Basosquamous- and Squamous-Cell Carcinoma Associated with Sun Exposure
by
Anamaria Grigore, Ana-Maria Oproiu, Ioana Iancu and Ioan-Petre Florescu
Curr. Oncol. 2024, 31(5), 2481-2487; https://doi.org/10.3390/curroncol31050185 (registering DOI) - 28 Apr 2024
Abstract
►▼
Show Figures
In recent years, there has been an observed increase in the frequency of cutaneous carcinoma, which correlates with sun exposure. This study aims to explore the variances of tumor characteristics and immune response markers among patients diagnosed with cutaneous squamous-cell carcinoma (SCC) and
[...] Read more.
In recent years, there has been an observed increase in the frequency of cutaneous carcinoma, which correlates with sun exposure. This study aims to explore the variances of tumor characteristics and immune response markers among patients diagnosed with cutaneous squamous-cell carcinoma (SCC) and basosquamous-cell carcinoma (BSC) with varying levels of sun exposure. The objective is to elucidate the potential influence of sun exposure on tumor progression and immune response in these types of carcinomas. We conducted a retrospective observational study that included 132 patients diagnosed with SCC and BSC. Participants were separated into high- and low-sun exposure groups. Tumor characteristics and immune response markers, including lymphocyte percentage (LY%), neutrophil-to-lymphocyte ratio (NLR), and lymphocyte-to-monocyte ratio (LMR), were assessed using the Mann–Whitney U test. Our findings revealed the interplay between sun exposure, inflammation, aging, and immune response. In 80% of cases, it was found that individuals had high sun exposure throughout their lifetime. Patients in the high sun exposure category had a significantly higher LY% than those with low sun exposure (24.22 ± 7.64 vs. 20.71 ± 8.10, p = 0.041). Also, the NLR was lower in patients with high sun exposure (3.08 ± 1.47 vs. 3.94 ± 2.43, p = 0.023). Regarding inflammatory markers, the erythrocyte sedimentation rate (ESR), LY%, NLR, and LMR showed significant differences between the two groups. Patients who were diagnosed with SCC had higher ESR values (p = 0.041), higher LY% (p = 0.037), higher NLR (p = 0.041), and lower LMR (p = 0.025). This study provides evidence supporting distinct tumor characteristics and immune response patterns in patients diagnosed with SCC and BSC with a high sun exposure history. These findings imply that sun exposure may contribute to tumor progression and influence the immune response in individuals with SCC and BSC.
Full article
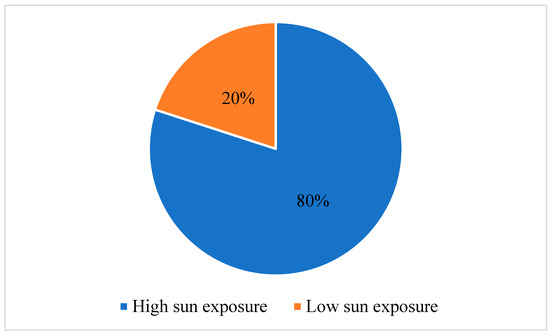
Figure 1
Open AccessPerspective
New Anticancer Drugs: Reliably Assessing “Value” While Addressing High Prices
by
David J. Stewart, John-Peter Bradford, Sandeep Sehdev, Tim Ramsay, Vishal Navani, Nigel S. B. Rawson, Di Maria Jiang, Joanna Gotfrit, Paul Wheatley-Price, Geoffrey Liu, Alan Kaplan, Silvana Spadafora, Shaun G. Goodman, Rebecca A. C. Auer and Gerald Batist
Curr. Oncol. 2024, 31(5), 2453-2480; https://doi.org/10.3390/curroncol31050184 (registering DOI) - 28 Apr 2024
Abstract
Countries face challenges in paying for new drugs. High prices are driven in part by exploding drug development costs, which, in turn, are driven by essential but excessive regulation. Burdensome regulation also delays drug development, and this can translate into thousands of life-years
[...] Read more.
Countries face challenges in paying for new drugs. High prices are driven in part by exploding drug development costs, which, in turn, are driven by essential but excessive regulation. Burdensome regulation also delays drug development, and this can translate into thousands of life-years lost. We need system-wide reform that will enable less expensive, faster drug development. The speed with which COVID-19 vaccines and AIDS therapies were developed indicates this is possible if governments prioritize it. Countries also differ in how they value drugs, and generally, those willing to pay more have better, faster access. Canada is used as an example to illustrate how “incremental cost-effectiveness ratios” (ICERs) based on measures such as gains in “quality-adjusted life-years” (QALYs) may be used to determine a drug’s value but are often problematic, imprecise assessments. Generally, ICER/QALY estimates inadequately consider the impact of patient crossover or long post-progression survival, therapy benefits in distinct subpopulations, positive impacts of the therapy on other healthcare or societal costs, how much governments willingly might pay for other things, etc. Furthermore, a QALY value should be higher for a lethal or uncommon disease than for a common, nonlethal disease. Compared to international comparators, Canada is particularly ineffective in initiating public funding for essential new medications. Addressing these disparities demands urgent reform.
Full article
(This article belongs to the Section Medical Oncology)
►▼
Show Figures
Figure 1
Open AccessArticle
DNA Quantity and Quality Comparisons between Cryopreserved and FFPE Tumors from Matched Pan-Cancer Samples
by
Jeffrey Okojie, Nikole O’Neal, Mackenzie Burr, Peyton Worley, Isaac Packer, DeLaney Anderson, Jack Davis, Bridger Kearns, Kaniz Fatema, Ken Dixon and Jared J. Barrott
Curr. Oncol. 2024, 31(5), 2441-2452; https://doi.org/10.3390/curroncol31050183 (registering DOI) - 28 Apr 2024
Abstract
►▼
Show Figures
Personalized cancer care requires molecular characterization of neoplasms. While the research community accepts frozen tissues as the gold standard analyte for molecular assays, the source of tissue for testing in clinical cancer care comes almost universally from formalin-fixed, paraffin-embedded tissue (FFPE). As newer
[...] Read more.
Personalized cancer care requires molecular characterization of neoplasms. While the research community accepts frozen tissues as the gold standard analyte for molecular assays, the source of tissue for testing in clinical cancer care comes almost universally from formalin-fixed, paraffin-embedded tissue (FFPE). As newer technologies emerge for DNA characterization that requires higher molecular weight DNA, it was necessary to compare the quality of DNA in terms of DNA length between FFPE and cryopreserved samples. We hypothesized that cryopreserved samples would yield higher quantity and superior quality DNA compared to FFPE samples. We analyzed DNA metrics by performing a head-to-head comparison between FFPE and cryopreserved samples from 38 human tumors representing various cancer types. DNA quantity and purity were measured by UV spectrophotometry, and DNA from cryopreserved tissue demonstrated a 4.2-fold increase in DNA yield per mg of tissue (p-value < 0.001). DNA quality was measured on a fragment microelectrophoresis analyzer, and again, DNA from cryopreserved tissue demonstrated a 223% increase in the DNA quality number and a 9-fold increase in DNA fragments > 40,000 bp (p-value < 0.0001). DNA from the cryopreserved tissues was superior to the DNA from FFPE samples in terms of DNA yield and quality.
Full article
Figure 1
Open AccessArticle
Real-World Analysis of Post-Progression Treatment Patterns and Outcomes for EGFR Mutation-Positive Patients Treated with First-Line Osimertinib
by
Amanda Jane Williams Gibson, Michelle Liane Dean, Ishjot Litt, Adrian Box, Winson Y. Cheung and Vishal Navani
Curr. Oncol. 2024, 31(5), 2427-2440; https://doi.org/10.3390/curroncol31050182 (registering DOI) - 26 Apr 2024
Abstract
Introduction: The use of osimertinib in the first-line (1L) setting is an effective treatment option for sensitizing EGFR-mutations (EGFRm+) and has significantly altered the standard of care practice for EGFRm+ disease in Canada. Unfortunately, acquired resistance to osimertinib is
[...] Read more.
Introduction: The use of osimertinib in the first-line (1L) setting is an effective treatment option for sensitizing EGFR-mutations (EGFRm+) and has significantly altered the standard of care practice for EGFRm+ disease in Canada. Unfortunately, acquired resistance to osimertinib is almost universal, and outcomes are disparate. Post-progression treatment patterns and the outcome of real-world Canadian EGFRm+ patients receiving 1L osimertinib were the focus of this retrospective review. Methods: The Glans-Look Lung Cancer Research database was used to identify and collect demographic, clinical, treatment, and outcome data on EGFRm+ patients who received 1L osimertinib in the Canadian province of Alberta between 2018 and 2022. Results: A total of 150 patients receiving 1L osimertinib were identified. In total, 86 developed progressive disease, with 56 (65%) continuing systemic therapy, 73% continuing osimertinib, and 27% switching to second-line (2L) systemic therapy. Patients were similar both in clinical characteristics at 1L osimertinib initiation and patterns of treatment failure at progression; those continuing 1L osimertinib post-progression had a longer time to progression (13.5 vs. 8.8 months, p = 0.05) and subsequent post-osimertinib initiation survival (34.7 vs. 22.8 months, p = 0.11). Conclusions: The continuation of osimertinib post-progression is an effective disease management strategy for select real-world EGFRm+ patients, providing continued clinical benefit, potentially due to different underlying disease pathogenesis.
Full article
(This article belongs to the Special Issue Clinical Management and Outcomes of Lung Cancer Patients)
►▼
Show Figures
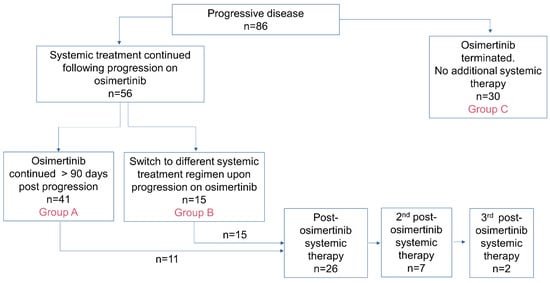
Figure 1
Open AccessCommentary
Developing an Adolescent and Young Adult Oncology Program in a Medium-Sized Canadian Centre: Lessons Learned
by
Ian Scott and Sapna Oberoi
Curr. Oncol. 2024, 31(5), 2420-2426; https://doi.org/10.3390/curroncol31050181 (registering DOI) - 26 Apr 2024
Abstract
The Adolescent and Young Adult (AYA) Program at CancerCare Manitoba (CCMB) has experienced tremendous growth since its inception. This report provides an overview of how the AYA program at CCMB was established and the crucial factors that led to its early accomplishments and
[...] Read more.
The Adolescent and Young Adult (AYA) Program at CancerCare Manitoba (CCMB) has experienced tremendous growth since its inception. This report provides an overview of how the AYA program at CCMB was established and the crucial factors that led to its early accomplishments and continued expansion. These factors included actions and decisions made at the individual and organizational level that helped lay a strong foundation for the program’s sustained success. We hope that some of these lessons learned can be adapted and implemented by other oncology agencies to improve the care outcomes and experiences of AYAs living with cancer.
Full article
(This article belongs to the Special Issue AYA Cancer Care and Support: Patient Perspectives, Programs, Practices, and Policy Change)
Open AccessArticle
Patients with Leptomeningeal Carcinomatosis and Hydrocephalus-Feasibility of Combined Ventriculoperitoneal Shunt and Reservoir Insertion for Intrathecal Chemotherapy
by
Matthias Schneider, Christian Wispel, Anna-Laura Potthoff, Muriel Heimann, Valeri Borger, Christina Schaub, Ulrich Herrlinger, Hartmut Vatter, Patrick Schuss and Niklas Schäfer
Curr. Oncol. 2024, 31(5), 2410-2419; https://doi.org/10.3390/curroncol31050180 (registering DOI) - 24 Apr 2024
Abstract
Therapeutic management of patients with leptomeningeal carcinomatosis (LC) may require treatment of concomitant hydrocephalus (HC) in addition to intrathecal chemotherapy (ITC). Ventriculoperitoneal shunts (VPS) equipped with a valve for manual deactivation of shunt function and a concomitant reservoir for application of ITC pose
[...] Read more.
Therapeutic management of patients with leptomeningeal carcinomatosis (LC) may require treatment of concomitant hydrocephalus (HC) in addition to intrathecal chemotherapy (ITC). Ventriculoperitoneal shunts (VPS) equipped with a valve for manual deactivation of shunt function and a concomitant reservoir for application of ITC pose an elegant solution to both problems. The present study evaluates indication, feasibility, and safety of such a modified shunt/reservoir design (mS/R). All patients with LC aged ≥ 18 years who had undergone mS/R implantation between 2013 and 2020 at the authors’ institution were further analyzed. ITC was indicated following the recommendation of the neuro-oncological tumor board and performed according to a standardized protocol. Sixteen patients with LC underwent mS/R implantation for subsequent ITC and concomitant treatment of HC. Regarding HC-related clinical symptoms, 69% of patients preoperatively exhibited lethargy, 38% cognitive impairment, and 38% (additional) visual disturbances. Postoperatively, 86% of patients achieved subjective improvement of HC-related symptoms. Overall, postoperative complications occurred in three patients (19%). No patient encountered cancer treatment-related complications. The present study describes a combination procedure consisting of a standard VPS-system and a standard reservoir for patients suffering from LC and HC. No cancer treatment-related complications occurred, indicating straightforward handling and thus safety.
Full article
(This article belongs to the Section Neuro-Oncology)
►▼
Show Figures
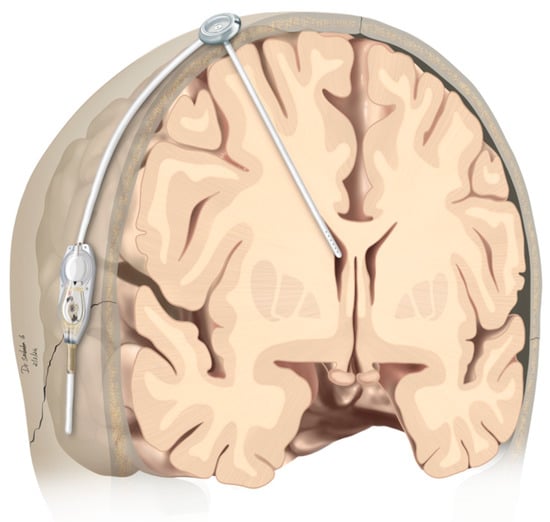
Figure 1
Open AccessFeature PaperArticle
Surgery Advances in Gynecologic Tumors: The Evolution and Outcomes of Robotic Surgery for Gynecologic Cancers in a Tertiary Center
by
David Knigin, Yoav Brezinov, Shannon Salvador, Susie Lau and Walter H. Gotlieb
Curr. Oncol. 2024, 31(5), 2400-2409; https://doi.org/10.3390/curroncol31050179 - 24 Apr 2024
Abstract
The integration of innovation into routine clinical practice is faced with many challenges. In 2007, we received the mandate to evaluate how the introduction of a robotic program in gynecologic oncology affected patient-centered care by studying its impact on clinical outcomes and hospital
[...] Read more.
The integration of innovation into routine clinical practice is faced with many challenges. In 2007, we received the mandate to evaluate how the introduction of a robotic program in gynecologic oncology affected patient-centered care by studying its impact on clinical outcomes and hospital resource utilization. Here we summarize the history and experience of developing a robotic surgery program for gynecologic cancers over 16 years. Analysis of the data indicates that robotic surgery improved perioperative patient clinical parameters, decreased blood loss, complications, and hospital stay, maintained the oncologic outcome, and is cost-effective, resulting in it becoming the dominant surgical approach in gynecologic oncology in a tertiary cancer care institution.
Full article
(This article belongs to the Special Issue Surgery Advances in Gynecologic Tumors)
►▼
Show Figures
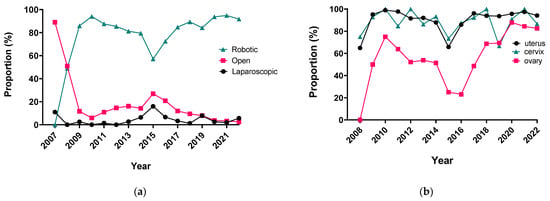
Figure 1
Open AccessCase Report
Mixed Adenosquamous Cell Carcinoma of the Prostate with Paired Sequencing on the Primary and Liver Metastasis
by
Emmanuella Oyogoa, Maya Sonpatki, Brian T. Brinkerhoff, Nicole Andeen, Haley Meyer, Christopher Ryan and Alexandra O. Sokolova
Curr. Oncol. 2024, 31(5), 2393-2399; https://doi.org/10.3390/curroncol31050178 - 24 Apr 2024
Abstract
This report aims to shed light on the intricate challenges encountered during the diagnosis and treatment of an uncommon variant of prostate cancer—mixed adenosquamous cell carcinoma of the prostate. Prostate cancers of this nature pose distinctive diagnostic and therapeutic dilemmas due to their
[...] Read more.
This report aims to shed light on the intricate challenges encountered during the diagnosis and treatment of an uncommon variant of prostate cancer—mixed adenosquamous cell carcinoma of the prostate. Prostate cancers of this nature pose distinctive diagnostic and therapeutic dilemmas due to their rarity and complex histological composition. We present a case of a 63-year-old man with metastatic prostate cancer, featuring adenocarcinoma with squamous cell differentiation, who underwent a multimodal treatment approach. The patient responded to first-line carboplatin, docetaxel, and androgen deprivation therapy, followed by androgen receptor pathway inhibitor (ARPI) maintenance. However, disease progression led to radiation therapy and a subsequent switch to Lutetium (177Lu) vipivotide tetraxetan after chemotherapy challenges. Comprehensive genetic profiling revealed shared mutations in the prostate and liver lesions, emphasizing the role of targeted therapies. Prostate-specific membrane antigen (PSMA)-targeted therapy resulted in a notable PSA decline. This case highlights the evolving treatment landscape for rare prostate cancers, integrating genetic insights for tailored interventions. In conclusion, squamous cell carcinoma (SCC) of the prostate is rare, emphasizing the imperative for enhanced comprehension in diagnosis and management. Our case suggests the potential efficacy of ARPI and PSMA-targeted therapies. Our findings advocate for a more nuanced approach to the management of this rare prostate cancer variant, leveraging genomic insights for personalized treatment strategies. This exploration serves as a foundation for further research and clinical considerations in addressing the challenges posed by mixed adenosquamous cell carcinoma of the prostate.
Full article
(This article belongs to the Topic Prostate Cancer: Symptoms, Diagnosis & Treatment - 2nd Volume)
►▼
Show Figures

Figure 1
Open AccessArticle
12-Month Trajectories of Health-Related Quality of Life Following Hospitalization in German Cancer Centers—A Secondary Data Analysis
by
Martin Eichler, Klaus Hönig, Corinna Bergelt, Hermann Faller, Imad Maatouk, Beate Hornemann, Barbara Stein, Martin Teufel, Ute Goerling, Yesim Erim, Franziska Geiser, Alexander Niecke, Bianca Senf and Joachim Weis
Curr. Oncol. 2024, 31(5), 2376-2392; https://doi.org/10.3390/curroncol31050177 (registering DOI) - 23 Apr 2024
Abstract
►▼
Show Figures
Patient-reported outcomes (PROs) offer a diverse array of potential applications within medical research and clinical practice. In comparative research, they can serve as tools for delineating the trajectories of health-related quality of life (HRQoL) across various cancer types. We undertook a secondary data
[...] Read more.
Patient-reported outcomes (PROs) offer a diverse array of potential applications within medical research and clinical practice. In comparative research, they can serve as tools for delineating the trajectories of health-related quality of life (HRQoL) across various cancer types. We undertook a secondary data analysis of a cohort of 1498 hospitalized cancer patients from 13 German cancer centers. We assessed the Physical and Mental Component Scores (PCS and MCS) of the 12-Item Short-Form Health Survey at baseline (t0), 6 (t1), and 12 months (t2), using multivariable generalized linear regression models. At baseline, the mean PCS and MCS values for all cancer patients were 37.1 and 44.3 points, respectively. We observed a significant improvement in PCS at t2 and in MCS at t1. The most substantial and significant improvements were noted among patients with gynecological cancers. We found a number of significant differences between cancer types at baseline, t1, and t2, with skin cancer patients performing best across all time points and lung cancer patients performing the worst. MCS trajectories showed less pronounced changes and differences between cancer types. Comparative analyses of HRQoL scores across different cancer types may serve as a valuable tool for enhancing health literacy, both among the general public and among cancer patients themselves.
Full article
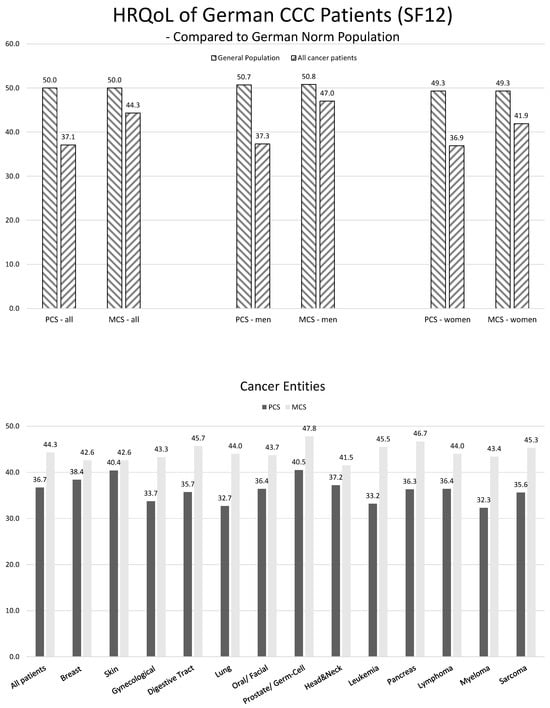
Figure 1
Open AccessReview
The Significance of Thyroid Hormone Receptors in Breast Cancer: A Hypothesis-Generating Narrative Review
by
Trinity Quan, Jessica Cockburn, Sukhbinder Dhesy-Thind, Anita Bane, Hon Leong, Christopher Geleff, Catherine Devion, Noor Ajel and Katarzyna J. Jerzak
Curr. Oncol. 2024, 31(5), 2364-2375; https://doi.org/10.3390/curroncol31050176 - 23 Apr 2024
Abstract
Background: Breast cancer (BC) is frequently diagnosed among Canadian women. While targeted therapies are available for most BC patients; treatment resistance is common and novel therapeutic targets are of interest. Thyroid hormones (TH) bound to thyroid hormone receptors (THR) influence cell proliferation and
[...] Read more.
Background: Breast cancer (BC) is frequently diagnosed among Canadian women. While targeted therapies are available for most BC patients; treatment resistance is common and novel therapeutic targets are of interest. Thyroid hormones (TH) bound to thyroid hormone receptors (THR) influence cell proliferation and differentiation; they are also involved in the growth and development of normal breast tissue. Evidence suggests that THRβ is a tumor suppressor in various solid tumors. Purpose: This narrative review discusses retrospective studies regarding the clinical relevance of THRβ as a potential prognostic biomarker and therapeutic target in BC. Methods: We consulted with an information specialist to develop a search strategy to find all literature related to THRα expression as a potential prognostic and therapeutic biomarker in breast cancer. The primary search was developed for Medline and translated to Embase. The searches were conducted on the Ovid platform on 18 August 2023. Results: Across seven retrospective studies identified, several have shown an association between higher THRβ1 expression with a lower risk of BC recurrence and with longer overall survival. Conclusions: Some evidence suggests that THRβ expression is associated with a lower risk of BC recurrence and death. Validation of THRβ as an independent prognostic biomarker and possible predictive biomarker of response to endocrine therapy and/or chemotherapy is of interest. Given that THRβ is upstream of the AKT/PI3K pathway, its potential as a predictive biomarker of response to AKT inhibitors and/or PI3K inhibitors may also be of value. Finally, the potential re-purposing of THRβ agonists as anti-cancer agents warrants investigation.
Full article
(This article belongs to the Special Issue Advanced Approaches to Breast Cancer Biomarkers)
►▼
Show Figures
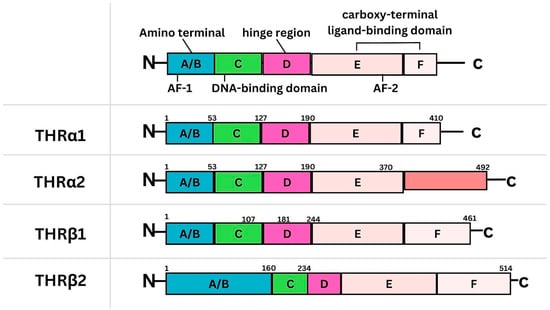
Figure 1
Open AccessReview
A Brief Overview of the Molecular Landscape of Myelodysplastic Neoplasms
by
Rami Abdulbaki and Sheeja T. Pullarkat
Curr. Oncol. 2024, 31(5), 2353-2363; https://doi.org/10.3390/curroncol31050175 - 23 Apr 2024
Abstract
Myelodysplastic neoplasm (MDS) is a heterogeneous group of clonal hematological disorders that originate from the hematopoietic and progenitor cells and present with cytopenias and morphologic dysplasia with a propensity to progress to bone marrow failure or acute myeloid leukemia (AML). Genetic evolution plays
[...] Read more.
Myelodysplastic neoplasm (MDS) is a heterogeneous group of clonal hematological disorders that originate from the hematopoietic and progenitor cells and present with cytopenias and morphologic dysplasia with a propensity to progress to bone marrow failure or acute myeloid leukemia (AML). Genetic evolution plays a critical role in the pathogenesis, progression, and clinical outcomes of MDS. This process involves the acquisition of genetic mutations in stem cells that confer a selective growth advantage, leading to clonal expansion and the eventual development of MDS. With the advent of next-generation sequencing (NGS) assays, an increasing number of molecular aberrations have been discovered in recent years. The knowledge of molecular events in MDS has led to an improved understanding of the disease process, including the evolution of the disease and prognosis, and has paved the way for targeted therapy. The 2022 World Health Organization (WHO) Classification and the International Consensus Classification (ICC) have incorporated the molecular signature into the classification system for MDS. In addition, specific germline mutations are associated with MDS development, especially in pediatrics and young adults. This article reviews the genetic abnormalities of MDS in adults with a brief review of germline predisposition syndromes.
Full article
(This article belongs to the Special Issue The Molecular Pathology of Myelodysplastic Syndromes)
►▼
Show Figures
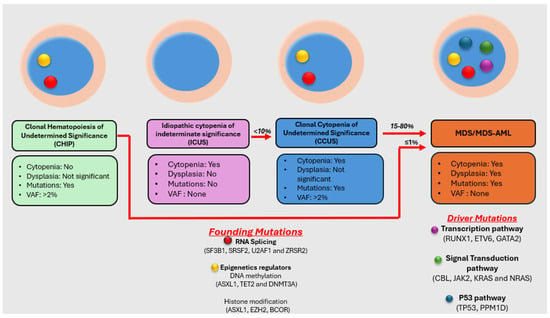
Figure 1
Open AccessReview
Gastro-Intestinal Symptoms in Palliative Care Patients
by
Golda Elisa Tradounsky
Curr. Oncol. 2024, 31(4), 2341-2352; https://doi.org/10.3390/curroncol31040174 - 21 Apr 2024
Abstract
This review of the palliation of various gastro-intestinal (GI) symptoms encountered in cancer patients is by no means exhaustive. Frequent symptoms such as constipation, nausea and vomiting, bowel obstructions, ascites and bleeds will be discussed, focusing on their assessment and most importantly, how
[...] Read more.
This review of the palliation of various gastro-intestinal (GI) symptoms encountered in cancer patients is by no means exhaustive. Frequent symptoms such as constipation, nausea and vomiting, bowel obstructions, ascites and bleeds will be discussed, focusing on their assessment and most importantly, how to control the associated symptoms. All of these symptoms and GI complications can significantly impact patients’ quality of life (QOL) and should be treated as quickly and aggressively as possible.
Full article
(This article belongs to the Special Issue 2023–2024 Article Series of the Canadian Association of General Practitioners in Oncology)
Open AccessArticle
The Impact of the Pandemic on the Quality of Colorectal and Anal Cancer Care, and 2-Year Clinical Outcomes
by
Melanie Powis, Rinku Sutradhar, Simron Singh, Shabbir Alibhai, Saidah Hack, Abed Baiad, Kevin Chen, Huaqi Li, Zuhal Mohmand and Monika K. Krzyzanowska
Curr. Oncol. 2024, 31(4), 2328-2340; https://doi.org/10.3390/curroncol31040173 - 19 Apr 2024
Abstract
We undertook a retrospective study to compare the quality of care delivered to a cohort of newly diagnosed adults with colon, rectal or anal cancer during the early phase of COVID-19 (02/20–12/20) relative to the same period in the year prior (the comparator
[...] Read more.
We undertook a retrospective study to compare the quality of care delivered to a cohort of newly diagnosed adults with colon, rectal or anal cancer during the early phase of COVID-19 (02/20–12/20) relative to the same period in the year prior (the comparator cohort), and examine the impact of the pandemic on 2-year disease progression and all-cause mortality. We observed poorer performance on a number of quality measures, such as approximately three times as many patients in the COVID-19 cohort experienced 30-day post-surgical readmission (10.5% vs. 3.6%; SD:0.27). Despite these differences, we observed no statistically significant adjusted associations between COVID-19 and time to either all-cause mortality (HR: 0.88, 95% CI: 0.61–1.27, p = 0.50) or disease progression (HR: 1.16, 95% CI: 0.82–1.64, p = 0.41). However, there was a substantial reduction in new patient consults during the early phase of COVID-19 (12.2% decrease), which appeared to disproportionally impact patients who traditionally experience sociodemographic disparities in access to care, given that the COVID-19 cohort skewed younger and there were fewer patients from neighborhoods with the highest Housing and Dwelling, ands Age and Labour Force marginalization quintiles. Future work is needed to understand the more downstream effects of COVID-19 related changes on cancer care to inform planning for future disruptions in care.
Full article
(This article belongs to the Section Gastrointestinal Oncology)
►▼
Show Figures
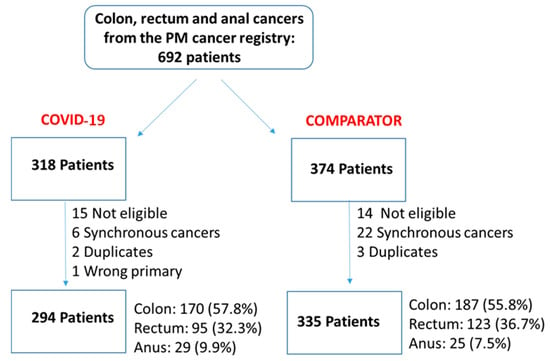
Figure 1
Open AccessReview
Antibody–Drug Conjugates in the Treatment of Genitourinary Cancers: An Updated Review of Data
by
Prathana Nathan, Adnan Rajeh, Meh Noor, Gabriel Boldt and Ricardo Fernandes
Curr. Oncol. 2024, 31(4), 2316-2327; https://doi.org/10.3390/curroncol31040172 - 19 Apr 2024
Abstract
The treatment landscape of genitourinary cancers has significantly evolved over the past few years. Renal cell carcinoma, bladder cancer, and prostate cancer are the most common genitourinary malignancies. Recent advancements have produced new targeted therapies, particularly antibody–drug conjugates (ADCs), due to a better
[...] Read more.
The treatment landscape of genitourinary cancers has significantly evolved over the past few years. Renal cell carcinoma, bladder cancer, and prostate cancer are the most common genitourinary malignancies. Recent advancements have produced new targeted therapies, particularly antibody–drug conjugates (ADCs), due to a better understanding of the underlying oncogenic factors and molecular mechanisms involved. ADCs function as a ‘drug delivery into the tumor’ system. They are composed of an antigen-directed antibody linked to a cytotoxic drug that releases cytotoxic components after binding to the tumor cell’s surface antigen. ADCs have been proven to be extremely promising in the treatment of several cancer types. For GU cancers, this novel treatment has only benefited patients with metastatic urothelial cancer (mUC). The rest of the GU cancer paradigm does not have any FDA-approved ADC treatment options available yet. In this study, we have thoroughly completed a narrative review of the current literature and summarized preclinical studies and clinical trials that evaluated the utility, activity, and toxicity of ADCs in GU cancers, the prospects of ADC development, and the ongoing clinical trials. Prospective clinical trials, retrospective studies, case reports, and scoping reviews were included.
Full article
(This article belongs to the Special Issue The Evolving Role of Antibody Drug Conjugates in Cancer Therapy)
►▼
Show Figures
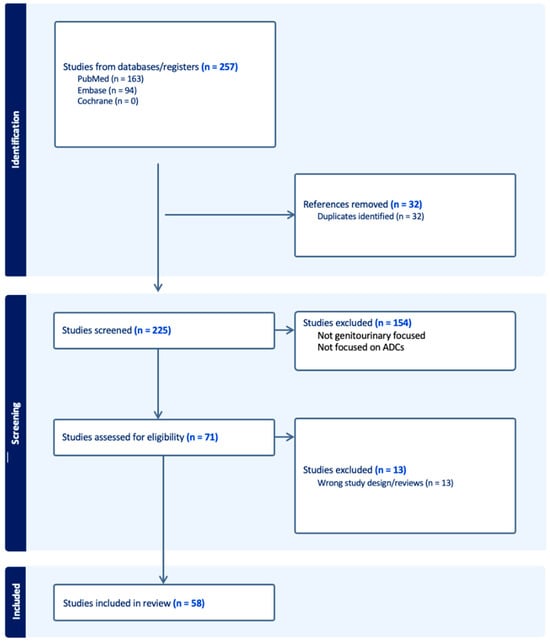
Figure 1

Journal Menu
► ▼ Journal Menu-
- Current Oncology Home
- Aims & Scope
- Editorial Board
- Reviewer Board
- Topical Advisory Panel
- Instructions for Authors
- Special Issues
- Topics
- Sections & Collections
- Article Processing Charge
- Indexing & Archiving
- Editor’s Choice Articles
- Most Cited & Viewed
- Journal Statistics
- Journal History
- Journal Awards
- Society Collaborations
- Conferences
- Editorial Office
Journal Browser
► ▼ Journal Browser-
arrow_forward_ios
Forthcoming issue
arrow_forward_ios Current issue - Volumes not published by MDPI
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Biology, Cancers, Current Oncology, Diseases, JCM, Pathogens
Pathogenetic, Diagnostic and Therapeutic Perspectives in Head and Neck Cancer
Topic Editors: Shun-Fa Yang, Ming-Hsien ChienDeadline: 20 June 2024
Topic in
Cancers, Cells, JCM, Radiation, Pharmaceutics, Applied Sciences, Nanomaterials, Current Oncology
Innovative Radiation Therapies
Topic Editors: Gérard Baldacchino, Eric Deutsch, Marie Dutreix, Sandrine Lacombe, Erika Porcel, Charlotte Robert, Emmanuelle Bourneuf, João Santos Sousa, Aurélien de la LandeDeadline: 30 June 2024
Topic in
Cancers, Diagnostics, JCM, Current Oncology, Gastrointestinal Disorders, Biomedicines
Hepatobiliary and Pancreatic Diseases: Novel Strategies of Diagnosis and Treatments
Topic Editors: Alessandro Coppola, Damiano Caputo, Roberta Angelico, Domenech Asbun, Chiara MazzarelliDeadline: 20 July 2024
Topic in
Cancers, Catalysts, Current Oncology, Plasma, Sci
Advances in Low-Temperature Plasma Cancer Therapy
Topic Editors: Michael Keidar, Li Lin, Dayun YanDeadline: 20 September 2024

Conferences
Special Issues
Special Issue in
Current Oncology
An Update on Surgical Treatment for Hepato-Pancreato-Biliary Cancers
Guest Editors: Nikolaos Machairas, Stylianos Kykalos, Dimitrios SchizasDeadline: 15 May 2024
Special Issue in
Current Oncology
Surgery Advances in Gynecologic Tumors
Guest Editor: Allan L. CovensDeadline: 31 May 2024
Special Issue in
Current Oncology
Physical Activity and Exercise in Cancer Care
Guest Editor: Melanie KeatsDeadline: 10 June 2024
Special Issue in
Current Oncology
Epidemiology and Risk Factors of Skin Cancer
Guest Editor: Leslie K. DennisDeadline: 30 June 2024
Topical Collections
Topical Collection in
Current Oncology
Editorial Board Members’ Collection Series: Contemporary Perioperative Concepts in Cancer Surgery
Collection Editors: Vijaya Gottumukkala, Jörg Kleeff
Topical Collection in
Current Oncology
Editorial Board Members’ Collection Series in "Exercise and Cancer Management"
Collection Editors: Linda Denehy, Ravi Mehrotra, Nicole Culos-Reed
Topical Collection in
Current Oncology
New Insights into Prostate Cancer Diagnosis and Treatment
Collection Editor: Sazan Rasul
Topical Collection in
Current Oncology
New Insights into Breast Cancer Diagnosis and Treatment
Collection Editors: Filippo Pesapane, Matteo Suter