Journal Description
Current Oncology
Current Oncology
is an international, peer-reviewed, open access journal published online by MDPI (from Volume 28 Issue 1-2021). Established in 1994, the journal represents a multidisciplinary medium for clinical oncologists to report and review progress in the management of this disease. The Canadian Association of Medical Oncologists (CAMO), the Canadian Association of Psychosocial Oncology (CAPO), the Canadian Association of General Practitioners in Oncology (CAGPO), the Cell Therapy Transplant Canada (CTTC), the Canadian Leukemia Study Group (CLSG) and others are affiliated with the journal and their members receive a discount on the article processing charges.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, SCIE (Web of Science), PubMed, MEDLINE, PMC, Embase, and other databases.
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 18 days after submission; acceptance to publication is undertaken in 2.8 days (median values for papers published in this journal in the second half of 2023).
- Recognition of Reviewers: APC discount vouchers, optional signed peer review, and reviewer names published annually in the journal.
Impact Factor:
2.6 (2022);
5-Year Impact Factor:
2.9 (2022)
Latest Articles
Minimally Invasive Conversion Surgery for Unresectable Gastric Cancer with Splenic Metastasis and Splenic Vein Tumor Thrombus: A Case Report
Curr. Oncol. 2024, 31(5), 2662-2669; https://doi.org/10.3390/curroncol31050201 - 08 May 2024
Abstract
While the importance of conversion surgery has increased with the development of systemic chemotherapy for gastric cancer (GC), reports of conversion surgery for patients with GC with distant metastasis and tumor thrombus are extremely scarce, and a definitive surgical strategy has yet to
[...] Read more.
While the importance of conversion surgery has increased with the development of systemic chemotherapy for gastric cancer (GC), reports of conversion surgery for patients with GC with distant metastasis and tumor thrombus are extremely scarce, and a definitive surgical strategy has yet to be established. Herein, we report a 67-year-old man with left abdominal pain referred to our hospital following a diagnosis of unresectable GC. Esophagogastroduodenoscopy and contrast-enhanced abdominal computed tomography (CT) revealed advanced GC with splenic metastasis. A splenic vein tumor thrombus (SVTT) and a continuous thrombus to the main trunk of the portal vein were detected. The patient was treated with anticoagulation therapy and systemic chemotherapy comprising S-1 and oxaliplatin. One year following chemotherapy initiation, a CT scan revealed progressive disease (PD); therefore, the chemotherapy regimen was switched to ramucirumab with paclitaxel. After 10 courses of chemotherapy resulting in primary tumor and SVTT shrinkage, the patient underwent laparoscopic total gastrectomy (LTG) and distal pancreaticosplenectomy (DPS). He was discharged without complications and remained alive 6 months postoperatively without recurrence. In summary, the wait-and-see approach was effective in a patient with GC with splenic metastasis and SVTT, ultimately leading to an R0 resection performed via LTG and DPS.
Full article
(This article belongs to the Topic Advances in Gastrointestinal and Liver Disease: From Physiological Mechanisms to Clinical Practice)
►
Show Figures
Open AccessArticle
Outcomes of Y90 Radioembolization for Hepatocellular Carcinoma in Patients Previously Treated with Transarterial Embolization
by
Ken Zhao, Sam Son, Anita Karimi, Brett Marinelli, Joseph P. Erinjeri, Erica S. Alexander, Vlasios S. Sotirchos, James J. Harding, Kevin C. Soares, Etay Ziv, Anne Covey, Constantinos T. Sofocleous and Hooman Yarmohammadi
Curr. Oncol. 2024, 31(5), 2650-2661; https://doi.org/10.3390/curroncol31050200 - 08 May 2024
Abstract
The aim of this study was to evaluate outcomes of transarterial radioembolization (TARE) for hepatocellular carcinoma (HCC) in patients previously treated with transarterial embolization (TAE). In this retrospective study, all HCC patients who received TARE from 1/2012 to 12/2022 for treatment of residual
[...] Read more.
The aim of this study was to evaluate outcomes of transarterial radioembolization (TARE) for hepatocellular carcinoma (HCC) in patients previously treated with transarterial embolization (TAE). In this retrospective study, all HCC patients who received TARE from 1/2012 to 12/2022 for treatment of residual or recurrent disease after TAE were identified. Overall survival (OS) was estimated using the Kaplan–Meier method. Univariate Cox regression was performed to determine significant predictors of OS after TARE. Twenty-one patients (median age 73.4 years, 18 male, 3 female) were included. Median dose to the perfused liver volume was 121 Gy (112–444, range), and 18/21 (85.7%) patients received 112–140 Gy. Median OS from time of HCC diagnosis was 32.9 months (19.4–61.4, 95% CI). Median OS after first TAE was 29.3 months (15.3–58.9, 95% CI). Median OS after first TARE was 10.6 months (6.8–27.0, 95% CI). ECOG performance status of 0 (p = 0.038), index tumor diameter < 4 cm (p = 0.022), and hepatic tumor burden < 25% (p = 0.018) were significant predictors of longer OS after TARE. TARE may provide a survival benefit for appropriately selected patients with HCC who have been previously treated with TAE.
Full article
(This article belongs to the Special Issue Radioembolization for Hepatocellular Carcinoma)
►▼
Show Figures
Figure 1
Open AccessCommunication
Incidence of Ophthalmological Complications in NF-1 Patients Treated with MEK Inhibitors
by
Lena Hummel, May Ameri, Shaikha Alqahtani, Zsila Sadighi and Nagham Al-Zubidi
Curr. Oncol. 2024, 31(5), 2644-2649; https://doi.org/10.3390/curroncol31050199 - 07 May 2024
Abstract
►▼
Show Figures
MEK inhibitors (MEKi) represent innovative and promising treatments for managing manifestations of neurofibromatosis type 1 (NF1). To mitigate potential ophthalmic side effects, such as MEKi-associated retinopathy (MEKAR), patients undergoing MEKi therapy routinely receive ophthalmology evaluations. Our study aims to assess the necessity of
[...] Read more.
MEK inhibitors (MEKi) represent innovative and promising treatments for managing manifestations of neurofibromatosis type 1 (NF1). To mitigate potential ophthalmic side effects, such as MEKi-associated retinopathy (MEKAR), patients undergoing MEKi therapy routinely receive ophthalmology evaluations. Our study aims to assess the necessity of this regular screening within a predominantly pediatric NF1 population by examining the occurrence of ocular adverse events (OAE). A retrospective study evaluated 45 NF1 patients receiving MEKi. Inclusion criteria included baseline and follow-up examinations following the initiation of MEKi therapy. At each assessment, a comprehensive eye evaluation was performed, comprising a dilated fundus examination, ocular coherence tomography of the macula and nerve fiber layer, and Humphrey visual field testing. Twenty-six patients, with an average age of 13 years (range 2–23 years) and an average follow-up duration of 413 days were included in the analysis. Three different MEKi were used: selumetinib (77%), trametinib (23%), and mirdametinib (4%). None of the patients experienced retinopathy at any point during the study. Some patients had pre-existing optic neuropathies (27%), but no instances of nerve changes occurred after commencing MEKi therapy. Four patients (15%) exhibited symptoms of dry eye, all of which were effectively managed with topical lubrication.
Full article
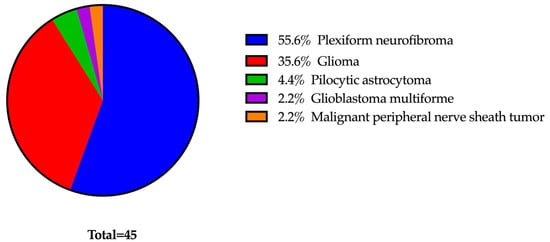
Figure 1
Open AccessFeature PaperArticle
Accelerated Fractionated Radiation Therapy for Localized Glottic Carcinoma
by
Tatsuji Mizukami, Kentaro Yamagishi, Masaki Tobikawa, Akira Nakazato, Hideharu Abe, Yuka Morita and Jun-ichi Saitoh
Curr. Oncol. 2024, 31(5), 2636-2643; https://doi.org/10.3390/curroncol31050198 - 06 May 2024
Abstract
Background: The aim of this study is to examine the outcomes of an accelerated fractionated irradiation for N0 glottic carcinoma. Methods: In this retrospective analysis, 29 patients with N0 glottic carcinoma treated by radiation therapy were enrolled. Thirteen patients had T1a disease, six
[...] Read more.
Background: The aim of this study is to examine the outcomes of an accelerated fractionated irradiation for N0 glottic carcinoma. Methods: In this retrospective analysis, 29 patients with N0 glottic carcinoma treated by radiation therapy were enrolled. Thirteen patients had T1a disease, six had T1b disease, and ten had T2 disease. A fractional dose of 2.1 Gy was administered to seven patients. The total doses were 65.1 and 67.2 Gy in four and three patients, respectively. A fractional dose of 2.25 Gy was administered to 22 patients. The total doses were 63 and 67.5 Gy in 21 patients and 1 patient with T2 disease, respectively. Additionally, 13 patients underwent the use of TS-1 (80–100 mg per day). Results: The median follow-up period was 33 months, and the 3-year local control rate was 95.6%. No patient had a lymph node or distant recurrence. As acute adverse events, grades 2 and 3 dermatitis were observed in 18 patients and 1 patient, and grades 2 and 3 mucositis were observed in 15 patients and 1 patient. As a late adverse event, one patient required tracheotomy because of laryngeal edema occurring. Conclusions: Accelerated fractionated irradiation may be an option in the radiation therapy of N0 glottic carcinoma because of its ability to shorten the treatment time.
Full article
(This article belongs to the Section Head and Neck Oncology)
►▼
Show Figures

Figure 1
Open AccessArticle
Understanding Elderly Chinese Cancer Patients in a Multicultural Clinical Setting: Embracing Mortality and Addressing Misperceptions of Vulnerability
by
Yvonne W. Leung, Enid W. Y. Kwong, Karen Lok Yi Wong, Jeremiah So, Frankie Poon, Terry Cheng, Eric Chen, Alex Molasiotis and Doris Howell
Curr. Oncol. 2024, 31(5), 2620-2635; https://doi.org/10.3390/curroncol31050197 - 05 May 2024
Abstract
Chinese patients face higher risks of gastrointestinal (GI) cancers and greater cancer-related deaths than Canadian-born patients. The older population encounters barriers to quality healthcare, impacting their well-being and survival. Previous studies highlighted Chinese immigrant perceptions of not requiring healthcare support. During the COVID-19
[...] Read more.
Chinese patients face higher risks of gastrointestinal (GI) cancers and greater cancer-related deaths than Canadian-born patients. The older population encounters barriers to quality healthcare, impacting their well-being and survival. Previous studies highlighted Chinese immigrant perceptions of not requiring healthcare support. During the COVID-19 pandemic, their underutilization of healthcare services garnered attention. The present study explores the experiences of older Chinese cancer patients to improve culturally sensitive cancer care. A total of twenty interviews carried out in Cantonese and Mandarin were conducted with Chinese immigrants, aged 60 or above, diagnosed with Stage 3 or 4 GI cancer. These interviews were transcribed verbatim, translated, and subjected to qualitative descriptive analysis. Among older Chinese immigrant patients, a phenomenon termed “Premature Acceptance: Normalizing Death and Dying” was observed. This involved four key themes: 1. acceptance and letting go, 2. family first, 3. self-sufficiency, and 4. barriers to supportive care. Participants displayed an early acceptance of their own mortality, prioritizing family prosperity over their own quality of life. Older Chinese patients normalize the reality of facing death amidst cancer. They adopt a pragmatic outlook, acknowledging life-saving treatments while willingly sacrificing their own support needs to ease family burdens. Efforts to enhance health literacy require culturally sensitive programs tailored to address language barriers and differing values among this population. A strengths-based approach emphasizing family support and practical aspects of care may help build resilience and improve symptom management, thereby enhancing their engagement with healthcare services.
Full article
(This article belongs to the Topic From Basic Research to a Clinical Perspective in Oncology)
►▼
Show Figures
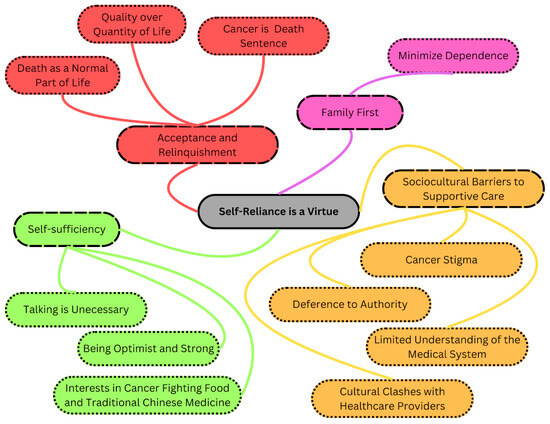
Figure 1
Open AccessArticle
Pan-Canadian Analysis of Practice Patterns in Small Cell Carcinoma of the Cervix: Insights from a Multidisciplinary Survey
by
Kevin Yijun Fan, Rania Chehade, Andrew Yuanbo Wang, Anjali Sachdeva, Helen J. MacKay and Amandeep S. Taggar
Curr. Oncol. 2024, 31(5), 2610-2619; https://doi.org/10.3390/curroncol31050196 - 03 May 2024
Abstract
►▼
Show Figures
Small-cell neuroendocrine carcinoma of the cervix (SCNECC) is a rare cancer with poor prognosis, with limited data to guide its treatment. The objective of this study was to evaluate practice patterns in the management of SCNECC. A 23-question online survey on management of
[...] Read more.
Small-cell neuroendocrine carcinoma of the cervix (SCNECC) is a rare cancer with poor prognosis, with limited data to guide its treatment. The objective of this study was to evaluate practice patterns in the management of SCNECC. A 23-question online survey on management of SCNECC was disseminated to Canadian gynecologic oncologists (GO), radiation oncologists (RO) and medical oncologists (MO). In total, 34 practitioners from eight provinces responded, including 17 GO, 13 RO and four MO. During staging and diagnosis, 74% of respondents used a trimodality imaging approach, and 85% tested for neuroendocrine markers. In early-stage (1A1-1B2) SCNECC, 87% of practitioners used a surgical-based approach with various adjuvant and neoadjuvant treatments. In locally advanced (1B3-IVA) SCNECC, 53% favored primary chemoradiation, with cisplatin and etoposide, with the remainder using surgical or radiation-based approaches. In metastatic and recurrent SCNECC, the most common first-line regimen was etoposide and platinum, and 63% of practitioners considered clinical trials in the first line setting or beyond. This survey highlights diverse practice patterns in the treatment of SCNECC. Interdisciplinary input is crucial to individualizing multimodality treatment, and there is a need for prospective trials and intergroup collaboration to define the optimal approach towards managing this rare cancer type.
Full article

Figure 1
Open AccessArticle
Brentuximab Vedotin Retreatment in Patients with Relapsed or Refractory Classical Hodgkin Lymphoma or Peripheral T-Cell Lymphoma: A Retrospective United States Claims Analysis
by
Dahlia Sano, Nicholas Liu, Scott Knowles, Joanna P. MacEwan, Shu Wang, Jenifer Wogen, Kristina S. Yu and Seung Tae Lee
Curr. Oncol. 2024, 31(5), 2598-2609; https://doi.org/10.3390/curroncol31050195 - 02 May 2024
Abstract
Brentuximab vedotin (BV) monotherapy (BV-M) and combination (BV-C) therapies are safe and effective for classical Hodgkin lymphoma (cHL) and CD30-expressing peripheral T-cell lymphomas (PTCLs). Although the sample sizes have been small (12–29 patients), in clinical studies, response rates of 53–88% have been reported
[...] Read more.
Brentuximab vedotin (BV) monotherapy (BV-M) and combination (BV-C) therapies are safe and effective for classical Hodgkin lymphoma (cHL) and CD30-expressing peripheral T-cell lymphomas (PTCLs). Although the sample sizes have been small (12–29 patients), in clinical studies, response rates of 53–88% have been reported for BV retreatment in patients with an initial BV response. We evaluated the real-world characteristics and treatment patterns of cHL/PTCL patients who received BV and were retreated in the United States. Symphony Health Patient Claims (11/2013–1/2022) were retrospectively analyzed to identify cHL/PTCL patients treated with BV and retreated with BV-M, BV-C, or non-BV therapy. Patient characteristics were described by retreatment, and predictors of BV-M retreatment were identified. Among the cHL and PTCL patients treated with BV (n = 6442 and 2472, respectively), 13% and 12%, respectively, were retreated with BV; the median times from initial BV to BV-M retreatment were 5 and 7 months, respectively; and the numbers of BV-M retreatment doses were 4 and 5, respectively. Among cHL patients, the predictors of BV-M retreatment were age (18–39 vs. ≥60 years), sex (women vs. men), and previous stem cell transplantation (yes vs. no). Among PTCL patients, the only predictor of BV-M retreatment was systemic anaplastic large-cell lymphoma subtype (yes vs. no). Real-world data support clinical study results suggesting earlier BV treatment be considered, as BV retreatment may be an option.
Full article
(This article belongs to the Section Hematology)
►▼
Show Figures

Figure 1
Open AccessFeature PaperArticle
Impact of an Inter-Professional Clinic on Pancreatic Cancer Outcomes: A Retrospective Cohort Study
by
Gordon Taylor Moffat, Zachary Coyne, Hamzeh Albaba, Kyaw Lwin Aung, Anna Dodd, Osvaldo Espin-Garcia, Shari Moura, Steven Gallinger, John Kim, Adriana Fraser, Shawn Hutchinson, Carol-Anne Moulton, Alice Wei, Ian McGilvray, Neesha Dhani, Raymond Jang, Elena Elimova, Malcolm Moore, Rebecca Prince and Jennifer Knox
Curr. Oncol. 2024, 31(5), 2589-2597; https://doi.org/10.3390/curroncol31050194 - 02 May 2024
Abstract
Background: Pancreatic ductal adenocarcinoma (PDAC) presents significant challenges in diagnosis, staging, and appropriate treatment. Furthermore, patients with PDAC often experience complex symptomatology and psychosocial implications that require multi-disciplinary and inter-professional supportive care management from health professionals. Despite these hurdles, the implementation of inter-professional
[...] Read more.
Background: Pancreatic ductal adenocarcinoma (PDAC) presents significant challenges in diagnosis, staging, and appropriate treatment. Furthermore, patients with PDAC often experience complex symptomatology and psychosocial implications that require multi-disciplinary and inter-professional supportive care management from health professionals. Despite these hurdles, the implementation of inter-professional clinic approaches showed promise in enhancing clinical outcomes. To assess the effectiveness of such an approach, we examined the impact of the Wallace McCain Centre for Pancreatic Cancer (WMCPC), an inter-professional clinic for patients with PDAC at the Princess Margaret Cancer Centre (PM). Methods: This retrospective cohort study included all patients diagnosed with PDAC who were seen at the PM before (July 2012–June 2014) and after (July 2014–June 2016) the establishment of the WMCPC. Standard therapies such as surgery, chemotherapy, and radiation therapy remained consistent across both time periods. The cohorts were compared in terms of survival rates, disease stage, referral patterns, time to treatment, symptoms, and the proportion of patients assessed and supported by nursing and allied health professionals. Results: A total of 993 patients were included in the review, comprising 482 patients pre-WMCPC and 511 patients post-WMCPC. In the multivariate analysis, adjusting for ECOG (Eastern Cooperative Oncology Group) and stage, it was found that post-WMCPC patients experienced longer median overall survival (mOS, HR 0.84, 95% CI 0.72–0.98, p = 0.023). Furthermore, the time from referral to initial consultation date decreased significantly from 13.4 to 8.8 days in the post-WMCPC cohort (p < 0.001), along with a reduction in the time from the first clinic appointment to biopsy (14 vs. 8 days, p = 0.022). Additionally, patient-reported well-being scores showed improvement in the post-WMCPC cohort (p = 0.02), and these patients were more frequently attended to by nursing and allied health professionals (p < 0.001). Conclusions: The implementation of an inter-professional clinic for patients diagnosed with PDAC led to improvements in overall survival, patient-reported well-being, time to initial assessment visit and pathological diagnosis, and symptom management. These findings advocate for the adoption of an inter-professional clinic model in the treatment of patients with PDAC.
Full article
(This article belongs to the Special Issue The 30th Anniversary of Current Oncology: Perspectives in Clinical Oncology Practice)
►▼
Show Figures
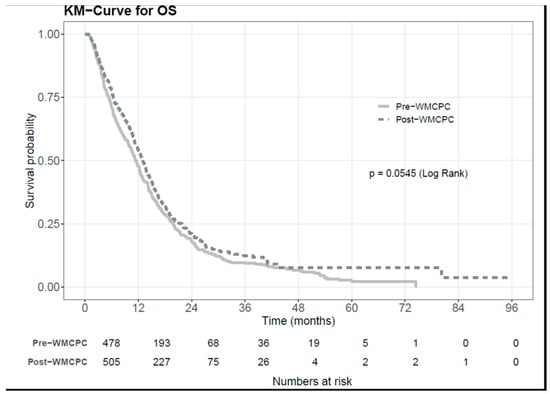
Figure 1
Open AccessCommentary
The Development and Impact of AYA Can—Canadian Cancer Advocacy: A Peer-Led Advocacy Organization for Adolescent and Young Adult Cancer in Canada
by
Chantale Thurston, Julie M. Deleemans, Jason Gisser, Emily Piercell, Vinesha Ramasamy and Perri R. Tutelman
Curr. Oncol. 2024, 31(5), 2582-2588; https://doi.org/10.3390/curroncol31050193 - 02 May 2024
Abstract
Adolescents and young adults (AYAs; 15–39 years) diagnosed with cancer face disparities in outcomes and survival. Patient advocacy organizations can play a pivotal role in advancing outcomes for underserved health conditions, such as AYA cancer. In 2018 a group of AYA patient advocates
[...] Read more.
Adolescents and young adults (AYAs; 15–39 years) diagnosed with cancer face disparities in outcomes and survival. Patient advocacy organizations can play a pivotal role in advancing outcomes for underserved health conditions, such as AYA cancer. In 2018 a group of AYA patient advocates founded AYA Canada (later renamed to “AYA Can—Canadian Cancer Advocacy”), a peer-led national organization aimed at improving the experiences and outcomes of Canadian AYAs affected by cancer. The aim of this article is to describe the development and impact of AYA Can. AYA Can was incorporated as a not-for-profit organization in 2021 and became a registered charity in 2023. Since 2018, AYA Can has established a thriving community of practice comprising nearly 300 patients, healthcare providers, researchers, and charitable organizations with an interest in advocacy for AYA cancer. Other activities have included advocacy at academic conferences and on scientific committees, collaboration with scientists to advance AYA cancer research, training the next generation of AYA patient advocates through a “patient ambassador program,” and developing a national resource hub to centralize knowledge and information on AYA cancer. Through its work to foster collaboration and amplify patient priorities on a national scale, AYA Can has become a leading voice for AYA cancer advocacy in Canada.
Full article
(This article belongs to the Special Issue AYA Cancer Care and Support: Patient Perspectives, Programs, Practices, and Policy Change)
►▼
Show Figures

Figure 1
Open AccessFeature PaperArticle
Income Disparities in Survival and Receipt of Neoadjuvant Chemotherapy and Pelvic Lymph Node Dissection for Muscle-Invasive Bladder Cancer
by
Ryan M. Antar, Vincent E. Xu, Oluwafolajimi Adesanya, Arthur Drouaud, Noah Longton, Olivia Gordon, Kirolos Youssef, Jad Kfouri, Sarah Azari, Sean Tafuri, Briana Goddard and Michael J. Whalen
Curr. Oncol. 2024, 31(5), 2566-2581; https://doi.org/10.3390/curroncol31050192 - 02 May 2024
Abstract
Background: Muscle-invasive bladder cancer (MIBC) is a potentially fatal disease, especially in the setting of locally advanced or node-positive disease. Adverse outcomes have also primarily been associated with low-income status, as has been reported in other cancers. While the adoption of neoadjuvant cisplatin-based
[...] Read more.
Background: Muscle-invasive bladder cancer (MIBC) is a potentially fatal disease, especially in the setting of locally advanced or node-positive disease. Adverse outcomes have also primarily been associated with low-income status, as has been reported in other cancers. While the adoption of neoadjuvant cisplatin-based chemotherapy (NAC) followed by radical cystectomy (RC) and pelvic lymph node dissection (PLND) has improved outcomes, these standard-of-care treatments may be underutilized in lower-income patients. We sought to investigate the economic disparities in NAC and PLND receipt and survival outcomes in MIBC. Methods: Utilizing the National Cancer Database, a retrospective cohort analysis of cT2-4N0-3M0 BCa patients with urothelial histology who underwent RC was conducted. The impact of income level on overall survival (OS) and the likelihood of receiving NAC and PLND was evaluated. Results: A total of 25,823 patients were included. This study found that lower-income patients were less likely to receive NAC and adequate PLND (≥15 LNs). Moreover, lower-income patients exhibited worse OS (Median OS 55.9 months vs. 68.2 months, p < 0.001). Our findings also demonstrated that higher income, treatment at academic facilities, and recent years of diagnosis were associated with an increased likelihood of receiving standard-of-care modalities and improved survival. Conclusions: Even after controlling for clinicodemographic variables, income independently influenced the receipt of standard MIBC treatments and survival. Our findings identify an opportunity to improve the quality of care for lower-income MIBC patients through concerted efforts to regionalize multi-modal urologic oncology care.
Full article
(This article belongs to the Special Issue Quality of Life and Satisfaction with Outcome among Cancer Survivors)
►▼
Show Figures

Figure 1
Open AccessReview
Best Practices for Managing Patients with Unresectable Metastatic Gastric and Gastroesophageal Junction Cancer in Canada
by
Stephanie Snow, Denise Gabrielson, Howard Lim, Mustapha Tehfe and Christine Brezden-Masley
Curr. Oncol. 2024, 31(5), 2552-2565; https://doi.org/10.3390/curroncol31050191 - 30 Apr 2024
Abstract
►▼
Show Figures
Gastric cancer (GC) is one of the most common types of cancer and is associated with relatively low survival rates. Despite its considerable burden, there is limited guidance for Canadian clinicians on the management of unresectable metastatic GC and gastroesophageal junction cancer (GEJC).
[...] Read more.
Gastric cancer (GC) is one of the most common types of cancer and is associated with relatively low survival rates. Despite its considerable burden, there is limited guidance for Canadian clinicians on the management of unresectable metastatic GC and gastroesophageal junction cancer (GEJC). Therefore, we aimed to discuss best practices and provide expert recommendations for patient management within the current Canadian unresectable GC and GEJC landscape. A multidisciplinary group of Canadian healthcare practitioners was assembled to develop expert recommendations via a working group. The often-rapid progression of unresectable GC and GEJC and the associated malnutrition have a significant impact on the patient’s quality of life and ability to tolerate treatment. Hence, recommendations include early diagnosis, identification of relevant biomarkers to improve personalized treatment, and relevant support to manage comorbidities. A multidisciplinary approach including early access to registered dietitians, personal support networks, and palliative care services, is needed to optimize possible outcomes for patients. Where possible, patients with unresectable GC and GEJC would benefit from access to clinical trials and innovative treatments.
Full article

Figure 1
Open AccessReview
Optimizing Access to Unrelated Donors in Canada: Re-Examining the Importance of Donor Factors on Outcomes Following Hematopoietic Cell Transplantation
by
Gaganvir Parmar, Matthew D. Seftel, Kathy Ganz, John Blake, Jelena L. Holovati and David S. Allan
Curr. Oncol. 2024, 31(5), 2542-2551; https://doi.org/10.3390/curroncol31050190 - 30 Apr 2024
Abstract
HLA-matched allogeneic hematopoietic cell transplantation (HCT) is a curative therapy for many patients. Unrelated HLA-matched donors are the most frequently used donor for HCT. When more than one donor transplant option is available, transplant centers can select donors based on non-HLA factors. With
[...] Read more.
HLA-matched allogeneic hematopoietic cell transplantation (HCT) is a curative therapy for many patients. Unrelated HLA-matched donors are the most frequently used donor for HCT. When more than one donor transplant option is available, transplant centers can select donors based on non-HLA factors. With improved ability to prevent and treat immune complications, such as graft-versus-host disease and infections, it may be possible to proceed more often using HLA-mismatched donors, allowing greater consideration of non-HLA factors, such as donor age, CMV serostatus, and ABO blood group matching, which have demonstrated important impacts on transplant outcomes. Additional factors to consider are donor availability rates and the usage of domestic donors to optimize outcomes. A review of non-HLA factors and considerations on the selection of optimal unrelated donors for HCT are provided within this updated current context.
Full article
(This article belongs to the Section Cell Therapy)
Open AccessArticle
Beauty Therapy to Support Psychosocial Recovery from Oncological Care: A Qualitative Research on the Lived Experience of Women with Breast Cancer Treated with Chemotherapy
by
Denise Vagnini, Massimo Maria Grassi, Francesco Valenti, Emilio Bombardieri and Emanuela Saita
Curr. Oncol. 2024, 31(5), 2527-2541; https://doi.org/10.3390/curroncol31050189 - 30 Apr 2024
Abstract
During the oncological care path, breast cancer patients treated with chemotherapy suffer from a number of psycho-physical changes, and appearance-related side effects are among the primary determinants of psychosocial impairment. Appropriate interventions are needed due to the fact that treatment-induced transformations have been
[...] Read more.
During the oncological care path, breast cancer patients treated with chemotherapy suffer from a number of psycho-physical changes, and appearance-related side effects are among the primary determinants of psychosocial impairment. Appropriate interventions are needed due to the fact that treatment-induced transformations have been associated with a decline in overall quality of life, interpersonal and sexual difficulties, and adverse effects on therapeutic adherence. In the framework of integrative oncology, beauty therapy is an affordable and straightforward intervention that could be used in the clinical management of breast cancer side effects. This study aims to comprehend the emotional and lived experiences of women undergoing chemotherapy after a brief beauty therapy intervention with licensed beauticians. The Interpretative Phenomenological Analysis was used as a methodological guideline. Sixteen women were purposefully recruited in a day hospital of a cancer unit, where the beauty therapy was implemented. At the end of the intervention, data were gathered using a semi-structured interview with open-ended questions. A thematic analysis was performed on verbatim transcriptions. Findings support the proposal of beauty therapy for patients undergoing chemotherapy. Assuming a relational viewpoint, beauty therapy could improve patients’ feelings about themselves and the way they feel about others, even if they do not declare a specific interest in their outward appearance.
Full article
(This article belongs to the Special Issue Body Image among Cancer Survivors: New Theoretical Frameworks and Psychological Interventions)
►▼
Show Figures
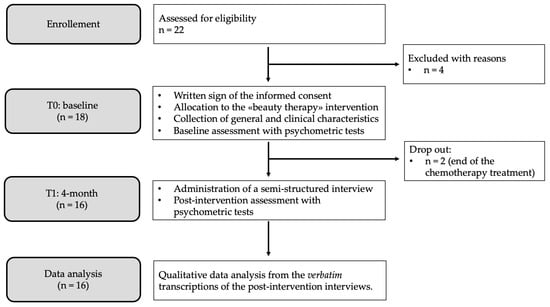
Figure 1
Open AccessReview
Impact of PET/CT Imaging with FDG in Locally Advanced Cervical Carcinoma—A Literature Review
by
Ottó Molnar, Oreste Mihai Straciuc, Simona Mihuțiu and Liviu Lazăr
Curr. Oncol. 2024, 31(5), 2508-2526; https://doi.org/10.3390/curroncol31050188 - 29 Apr 2024
Abstract
Positron emission tomography (PET) and computed tomography (CT) have evolved as a pivotal diagnostic modality in the field of oncology. With its increasing application in staging and ready availability, it becomes imperative for committed radiation oncologists to possess a complete analysis and understanding
[...] Read more.
Positron emission tomography (PET) and computed tomography (CT) have evolved as a pivotal diagnostic modality in the field of oncology. With its increasing application in staging and ready availability, it becomes imperative for committed radiation oncologists to possess a complete analysis and understanding of integration of molecular imaging, which can be helpful for radiation planning, while also acknowledging its possible limitations and challenges. A significant obstacle lies in the synthesis and design of tumor-specific bmolecules for diagnosing and treating cancer. The utilization of radiation in medical biochemistry and biotechnology, encompassing diagnosis, therapy, and control of biological systems, is encapsulated under the umbrella term “nuclear medicine.” Notably, the application of various radioisotopes in pharmaceutics has garnered significant attention, particularly in the realm of delivery systems for drugs, DNA, and imaging agents. The present article provides a comprehensive review of use of novel techniques PET and CT with major positron-emitting radiopharmaceuticals currently in progress or utilized in clinical practice with their integration into imaging and radiation therapy.
Full article
(This article belongs to the Section Gynecologic Oncology)
Open AccessReview
Sublobar Resection of Non-Small-Cell Lung Cancer: Wedge Resection vs. Segmentectomy
by
Kyeong Ri Yu and Walker A. Julliard
Curr. Oncol. 2024, 31(5), 2497-2507; https://doi.org/10.3390/curroncol31050187 - 29 Apr 2024
Abstract
Lung cancer is the most common cause of cancer death. The mainstay treatment for non-small-cell lung cancer (NSCLC), particularly in the early stages, is surgical resection. Traditionally, lobectomy has been considered the gold-standard technique. Sublobar resection includes segmentectomy and wedge resection. Compared to
[...] Read more.
Lung cancer is the most common cause of cancer death. The mainstay treatment for non-small-cell lung cancer (NSCLC), particularly in the early stages, is surgical resection. Traditionally, lobectomy has been considered the gold-standard technique. Sublobar resection includes segmentectomy and wedge resection. Compared to lobectomy, these procedures have been viewed as a compromise procedure, reserved for those with poor cardiopulmonary function or who are poor surgical candidates for other reasons. However, with the advances in imaging and surgical techniques, the subject of sublobar resection as a curative treatment is being revisited. Many studies have now shown segmentectomy to be equivalent to lobectomy in patients with small (<2.0 cm), peripheral NSCLC. However, there is a mix of evidence when it comes to wedge resection and its suitability as a curative procedure. At this time, until more data can be found, segmentectomy should be considered before wedge resection for patients with early-stage NSCLC.
Full article
(This article belongs to the Special Issue New Advances in the Treatment of Resectable Non-small Cell Lung Cancer)
Open AccessArticle
Evaluation of Unsolicited Feedback from Patients with Cancer and Their Families as a Strategy to Improve Cancer Care Delivery
by
Parvaneh Fallah, Lucas Clemons, Michelle Bradbury, Lisa Vandermeer, Mark Clemons, Julie Renaud and Marie-France Savard
Curr. Oncol. 2024, 31(5), 2488-2496; https://doi.org/10.3390/curroncol31050186 - 28 Apr 2024
Abstract
Background: Unsolicited patient feedback (compliments and complaints) should allow the healthcare system to address and improve individual and overall patient, family, and staff experiences. We evaluated feedback at a tertiary cancer centre to identify potential areas for optimizing care delivery. Methods: unsolicited feedback
[...] Read more.
Background: Unsolicited patient feedback (compliments and complaints) should allow the healthcare system to address and improve individual and overall patient, family, and staff experiences. We evaluated feedback at a tertiary cancer centre to identify potential areas for optimizing care delivery. Methods: unsolicited feedback submitted to the Patient Relations Department, relating to the Divisions of Medical and Radiation Oncology, at the Ottawa Hospital, was analyzed. Results: Of 580 individual reports submitted from 2016 to 2022, patient demographics were available for 97% (563/580). Median patient age was 65 years (range 17–101), and 53% (301/563) were female. The most common cancer types were breast (127/545, 23%) and gastrointestinal (119/545, 22%) malignancies, and most (64%, 311/486) patients had metastatic disease. Feedback was submitted mainly by patients (291/579, 50%), and predominantly negative (489/569, 86%). The main reasons for complaints included: communication (29%, 162/566) and attitude/conduct of care (28%, 159/566). While feedback rates were initially stable, an increase occurred from 2019 to 2021. Conclusions: Unsolicited feedback remains mostly negative, and relates to physician communication. If we are to drive meaningful changes in care delivery, more standardized means of assessing feedback and implementation strategies are needed. In addition, in an era of increased healthcare provider burnout, strategies to enhance formal positive feedback are also warranted.
Full article
(This article belongs to the Special Issue The 30th Anniversary of Current Oncology: Perspectives in Clinical Oncology Practice)
►▼
Show Figures
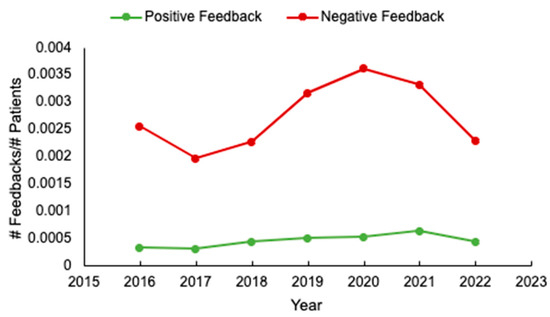
Figure 1
Open AccessCommunication
The Immune Response of Cutaneous Basosquamous- and Squamous-Cell Carcinoma Associated with Sun Exposure
by
Anamaria Grigore, Ana-Maria Oproiu, Ioana Iancu and Ioan-Petre Florescu
Curr. Oncol. 2024, 31(5), 2481-2487; https://doi.org/10.3390/curroncol31050185 - 28 Apr 2024
Abstract
►▼
Show Figures
In recent years, there has been an observed increase in the frequency of cutaneous carcinoma, which correlates with sun exposure. This study aims to explore the variances of tumor characteristics and immune response markers among patients diagnosed with cutaneous squamous-cell carcinoma (SCC) and
[...] Read more.
In recent years, there has been an observed increase in the frequency of cutaneous carcinoma, which correlates with sun exposure. This study aims to explore the variances of tumor characteristics and immune response markers among patients diagnosed with cutaneous squamous-cell carcinoma (SCC) and basosquamous-cell carcinoma (BSC) with varying levels of sun exposure. The objective is to elucidate the potential influence of sun exposure on tumor progression and immune response in these types of carcinomas. We conducted a retrospective observational study that included 132 patients diagnosed with SCC and BSC. Participants were separated into high- and low-sun exposure groups. Tumor characteristics and immune response markers, including lymphocyte percentage (LY%), neutrophil-to-lymphocyte ratio (NLR), and lymphocyte-to-monocyte ratio (LMR), were assessed using the Mann–Whitney U test. Our findings revealed the interplay between sun exposure, inflammation, aging, and immune response. In 80% of cases, it was found that individuals had high sun exposure throughout their lifetime. Patients in the high sun exposure category had a significantly higher LY% than those with low sun exposure (24.22 ± 7.64 vs. 20.71 ± 8.10, p = 0.041). Also, the NLR was lower in patients with high sun exposure (3.08 ± 1.47 vs. 3.94 ± 2.43, p = 0.023). Regarding inflammatory markers, the erythrocyte sedimentation rate (ESR), LY%, NLR, and LMR showed significant differences between the two groups. Patients who were diagnosed with SCC had higher ESR values (p = 0.041), higher LY% (p = 0.037), higher NLR (p = 0.041), and lower LMR (p = 0.025). This study provides evidence supporting distinct tumor characteristics and immune response patterns in patients diagnosed with SCC and BSC with a high sun exposure history. These findings imply that sun exposure may contribute to tumor progression and influence the immune response in individuals with SCC and BSC.
Full article
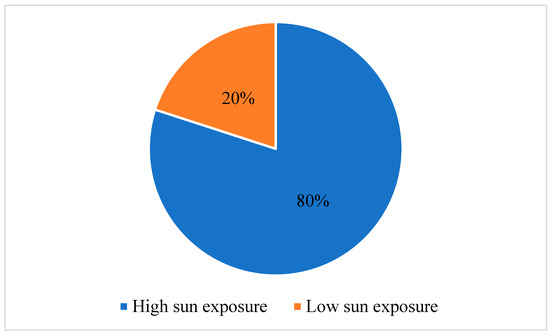
Figure 1
Open AccessPerspective
New Anticancer Drugs: Reliably Assessing “Value” While Addressing High Prices
by
David J. Stewart, John-Peter Bradford, Sandeep Sehdev, Tim Ramsay, Vishal Navani, Nigel S. B. Rawson, Di Maria Jiang, Joanna Gotfrit, Paul Wheatley-Price, Geoffrey Liu, Alan Kaplan, Silvana Spadafora, Shaun G. Goodman, Rebecca A. C. Auer and Gerald Batist
Curr. Oncol. 2024, 31(5), 2453-2480; https://doi.org/10.3390/curroncol31050184 - 28 Apr 2024
Abstract
Countries face challenges in paying for new drugs. High prices are driven in part by exploding drug development costs, which, in turn, are driven by essential but excessive regulation. Burdensome regulation also delays drug development, and this can translate into thousands of life-years
[...] Read more.
Countries face challenges in paying for new drugs. High prices are driven in part by exploding drug development costs, which, in turn, are driven by essential but excessive regulation. Burdensome regulation also delays drug development, and this can translate into thousands of life-years lost. We need system-wide reform that will enable less expensive, faster drug development. The speed with which COVID-19 vaccines and AIDS therapies were developed indicates this is possible if governments prioritize it. Countries also differ in how they value drugs, and generally, those willing to pay more have better, faster access. Canada is used as an example to illustrate how “incremental cost-effectiveness ratios” (ICERs) based on measures such as gains in “quality-adjusted life-years” (QALYs) may be used to determine a drug’s value but are often problematic, imprecise assessments. Generally, ICER/QALY estimates inadequately consider the impact of patient crossover or long post-progression survival, therapy benefits in distinct subpopulations, positive impacts of the therapy on other healthcare or societal costs, how much governments willingly might pay for other things, etc. Furthermore, a QALY value should be higher for a lethal or uncommon disease than for a common, nonlethal disease. Compared to international comparators, Canada is particularly ineffective in initiating public funding for essential new medications. Addressing these disparities demands urgent reform.
Full article
(This article belongs to the Section Medical Oncology)
►▼
Show Figures
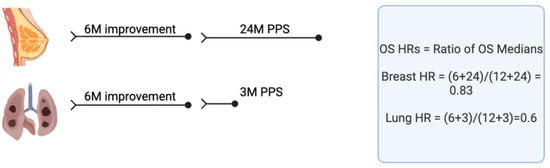
Figure 1
Open AccessArticle
DNA Quantity and Quality Comparisons between Cryopreserved and FFPE Tumors from Matched Pan-Cancer Samples
by
Jeffrey Okojie, Nikole O’Neal, Mackenzie Burr, Peyton Worley, Isaac Packer, DeLaney Anderson, Jack Davis, Bridger Kearns, Kaniz Fatema, Ken Dixon and Jared J. Barrott
Curr. Oncol. 2024, 31(5), 2441-2452; https://doi.org/10.3390/curroncol31050183 - 28 Apr 2024
Abstract
►▼
Show Figures
Personalized cancer care requires molecular characterization of neoplasms. While the research community accepts frozen tissues as the gold standard analyte for molecular assays, the source of tissue for testing in clinical cancer care comes almost universally from formalin-fixed, paraffin-embedded tissue (FFPE). As newer
[...] Read more.
Personalized cancer care requires molecular characterization of neoplasms. While the research community accepts frozen tissues as the gold standard analyte for molecular assays, the source of tissue for testing in clinical cancer care comes almost universally from formalin-fixed, paraffin-embedded tissue (FFPE). As newer technologies emerge for DNA characterization that requires higher molecular weight DNA, it was necessary to compare the quality of DNA in terms of DNA length between FFPE and cryopreserved samples. We hypothesized that cryopreserved samples would yield higher quantity and superior quality DNA compared to FFPE samples. We analyzed DNA metrics by performing a head-to-head comparison between FFPE and cryopreserved samples from 38 human tumors representing various cancer types. DNA quantity and purity were measured by UV spectrophotometry, and DNA from cryopreserved tissue demonstrated a 4.2-fold increase in DNA yield per mg of tissue (p-value < 0.001). DNA quality was measured on a fragment microelectrophoresis analyzer, and again, DNA from cryopreserved tissue demonstrated a 223% increase in the DNA quality number and a 9-fold increase in DNA fragments > 40,000 bp (p-value < 0.0001). DNA from the cryopreserved tissues was superior to the DNA from FFPE samples in terms of DNA yield and quality.
Full article
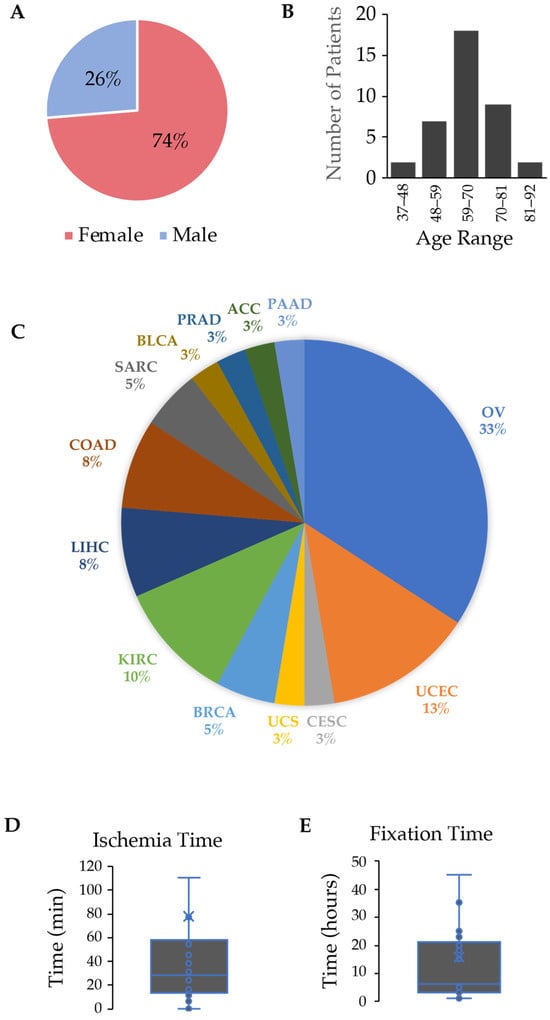
Figure 1
Open AccessArticle
Real-World Analysis of Post-Progression Treatment Patterns and Outcomes for EGFR Mutation-Positive Patients Treated with First-Line Osimertinib
by
Amanda Jane Williams Gibson, Michelle Liane Dean, Ishjot Litt, Adrian Box, Winson Y. Cheung and Vishal Navani
Curr. Oncol. 2024, 31(5), 2427-2440; https://doi.org/10.3390/curroncol31050182 - 26 Apr 2024
Abstract
Introduction: The use of osimertinib in the first-line (1L) setting is an effective treatment option for sensitizing EGFR-mutations (EGFRm+) and has significantly altered the standard of care practice for EGFRm+ disease in Canada. Unfortunately, acquired resistance to osimertinib is
[...] Read more.
Introduction: The use of osimertinib in the first-line (1L) setting is an effective treatment option for sensitizing EGFR-mutations (EGFRm+) and has significantly altered the standard of care practice for EGFRm+ disease in Canada. Unfortunately, acquired resistance to osimertinib is almost universal, and outcomes are disparate. Post-progression treatment patterns and the outcome of real-world Canadian EGFRm+ patients receiving 1L osimertinib were the focus of this retrospective review. Methods: The Glans-Look Lung Cancer Research database was used to identify and collect demographic, clinical, treatment, and outcome data on EGFRm+ patients who received 1L osimertinib in the Canadian province of Alberta between 2018 and 2022. Results: A total of 150 patients receiving 1L osimertinib were identified. In total, 86 developed progressive disease, with 56 (65%) continuing systemic therapy, 73% continuing osimertinib, and 27% switching to second-line (2L) systemic therapy. Patients were similar both in clinical characteristics at 1L osimertinib initiation and patterns of treatment failure at progression; those continuing 1L osimertinib post-progression had a longer time to progression (13.5 vs. 8.8 months, p = 0.05) and subsequent post-osimertinib initiation survival (34.7 vs. 22.8 months, p = 0.11). Conclusions: The continuation of osimertinib post-progression is an effective disease management strategy for select real-world EGFRm+ patients, providing continued clinical benefit, potentially due to different underlying disease pathogenesis.
Full article
(This article belongs to the Special Issue Clinical Management and Outcomes of Lung Cancer Patients)
►▼
Show Figures
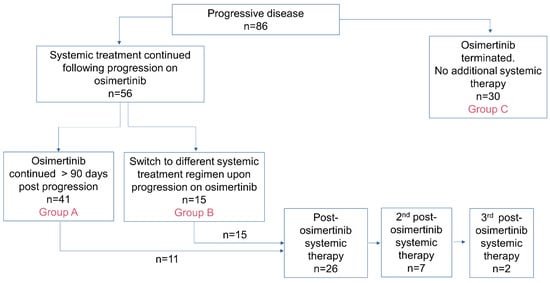
Figure 1

Journal Menu
► ▼ Journal Menu-
- Current Oncology Home
- Aims & Scope
- Editorial Board
- Reviewer Board
- Topical Advisory Panel
- Instructions for Authors
- Special Issues
- Topics
- Sections & Collections
- Article Processing Charge
- Indexing & Archiving
- Editor’s Choice Articles
- Most Cited & Viewed
- Journal Statistics
- Journal History
- Journal Awards
- Society Collaborations
- Conferences
- Editorial Office
Journal Browser
► ▼ Journal Browser-
arrow_forward_ios
Forthcoming issue
arrow_forward_ios Current issue - Volumes not published by MDPI
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Biology, Cancers, Current Oncology, Diseases, JCM, Pathogens
Pathogenetic, Diagnostic and Therapeutic Perspectives in Head and Neck Cancer
Topic Editors: Shun-Fa Yang, Ming-Hsien ChienDeadline: 20 June 2024
Topic in
Cancers, Cells, JCM, Radiation, Pharmaceutics, Applied Sciences, Nanomaterials, Current Oncology
Innovative Radiation Therapies
Topic Editors: Gérard Baldacchino, Eric Deutsch, Marie Dutreix, Sandrine Lacombe, Erika Porcel, Charlotte Robert, Emmanuelle Bourneuf, João Santos Sousa, Aurélien de la LandeDeadline: 30 June 2024
Topic in
Cancers, Diagnostics, JCM, Current Oncology, Gastrointestinal Disorders, Biomedicines
Hepatobiliary and Pancreatic Diseases: Novel Strategies of Diagnosis and Treatments
Topic Editors: Alessandro Coppola, Damiano Caputo, Roberta Angelico, Domenech Asbun, Chiara MazzarelliDeadline: 20 July 2024
Topic in
Cancers, Catalysts, Current Oncology, Plasma, Sci
Advances in Low-Temperature Plasma Cancer Therapy
Topic Editors: Michael Keidar, Li Lin, Dayun YanDeadline: 20 September 2024

Conferences
Special Issues
Special Issue in
Current Oncology
An Update on Surgical Treatment for Hepato-Pancreato-Biliary Cancers
Guest Editors: Stylianos Kykalos, Dimitrios Schizas, Nikolaos MachairasDeadline: 15 May 2024
Special Issue in
Current Oncology
Surgery Advances in Gynecologic Tumors
Guest Editor: Allan L. CovensDeadline: 31 May 2024
Special Issue in
Current Oncology
Physical Activity and Exercise in Cancer Care
Guest Editor: Melanie KeatsDeadline: 10 June 2024
Special Issue in
Current Oncology
Epidemiology and Risk Factors of Skin Cancer
Guest Editor: Leslie K. DennisDeadline: 30 June 2024
Topical Collections
Topical Collection in
Current Oncology
Editorial Board Members’ Collection Series: Contemporary Perioperative Concepts in Cancer Surgery
Collection Editors: Jörg Kleeff, Vijaya Gottumukkala
Topical Collection in
Current Oncology
Editorial Board Members’ Collection Series in "Exercise and Cancer Management"
Collection Editors: Ravi Mehrotra, Nicole Culos-Reed, Linda Denehy
Topical Collection in
Current Oncology
New Insights into Prostate Cancer Diagnosis and Treatment
Collection Editor: Sazan Rasul
Topical Collection in
Current Oncology
New Insights into Breast Cancer Diagnosis and Treatment
Collection Editors: Matteo Suter, Filippo Pesapane